Conductive Paste, The First Experience
Objective: Evaluating conductive characteristics of graphite powder to make conductive ink or paste
Nowadays using conductive inks and pastes consumption has risen to a great extent. Wearables generally need conductive paths on flexible polymers can be likened to copper on fiber glass sheets (FR4) to build up an electrical circuit. Use of fine grain or nano metal inks like Ag or Ni solve the problem but they are generally pricey and cannot be used by everyone. In this experience I use graphite powder to make some pastes. Their grain size is not in order of nano materials so they are not good candidate for nano ink. Anyway they can be used as ‘conductive paste’ or something along those lines.
First of all it is worth mentioning that graphite powder is sold in two forms: low sulfur and high sulfur. In this experiment the role of sulfur needs to be defined and higher conductivity material must be used to make the conductive paste. One must bear in mind that grain size of graphite is a crucial parameter as the higher grains lead to paste with sand like grains and not proper for silk screen or deposition on surfaces. Below is the image of two types of graphite powder.

Here is the look on the watch glass. Low sulfur type is greasy while the high sulfur type is not. It is hard to clean low sulfur one from hands by liquid soap and only thinner can help. 80 micrometer mesh is improper for making ink let alone 320 micro meter mesh for high sulfur type. Fine grain type are so pricey and out of reach.

In order to heighten the conductivity and also adhesivity of mixture, sodium silicate or metasilicate (Na2SiO3) is used. This cheap material is also called “glass water” and can be found easily on chemical stores or even department stores as glass water or liquid water. I bought 1Kg for several dollars from a local chemical store. Here is the look.

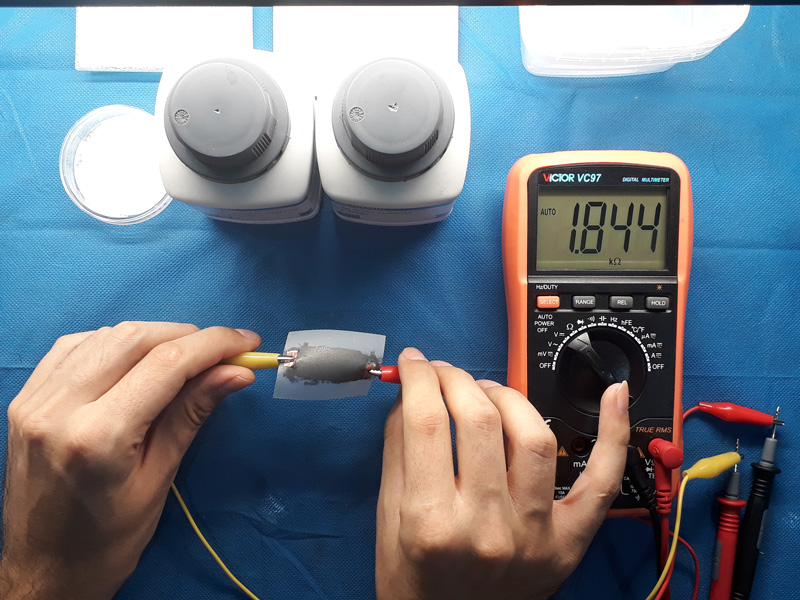
As a first experience, 1 gram of each type of graphite is added to 1 gram of sodium metasilicate powder and then distilled water is added to make a paste. Then two samples widened on the paper substrate and were left to dry. After fully drying of samples, it is time to measure the electrical resistance. The right layer is for low sulfur and the left is for the high one.


The measured resistance denotes the higher conductivity for low sulfur type though it is not a good criterion as the thickness of the layer is not equal, just a rough test. It is so hard to test these two samples even under a mold. as the compression can easily make layers compact and changes the conductivity. Anyway the right sample is chosen for further investigation. Besides lower mesh size can make it a better choice for deposition on surfaces. Two copper plates as connection electrode are put at both ends of samples paper deposited with graphite paste to connect crocodile clips. The results are as below.
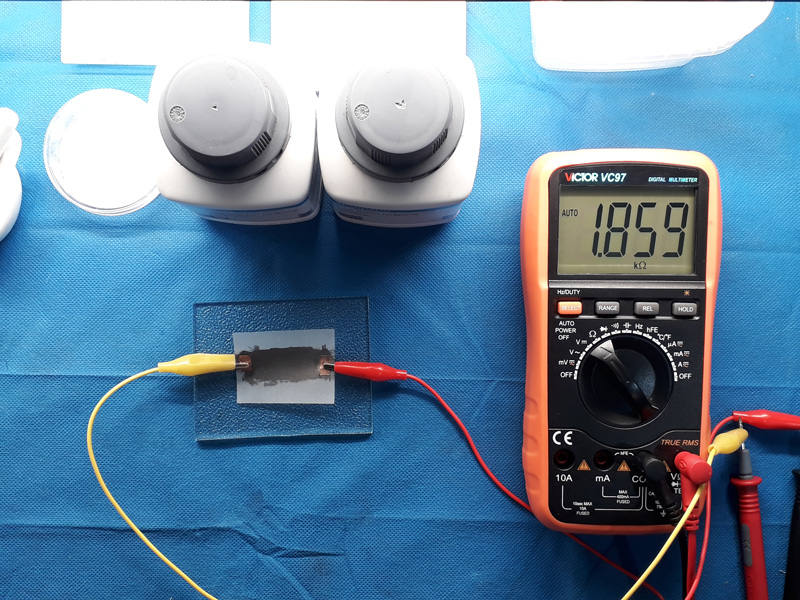
Another test was conducted bending the paper substrate. Results show nearly 10% increase in resistance value promising the use of the graphite papers for flex sensors.

The same experiment was done twisting the paper substrate. It is seen that no practical change in resistance has been made.